What is Lattice Energy?
Ahead of discussing how to calculate lattice energy, let’s define it. Lattice energy also known as lattice enthalpy required to separate a mole of an ionic solid into gaseous ions or the energy that must be supplied to one mole of an ionic crystal in order to separate it into gaseous ions in a vacuum via an endothermic process.
Lattice energies are important in predicting the solubility and stability of ionic solids in water.
This type of energy cannot be measured empirically, but can be calculated using electrostatics or estimated using the Born Haber cycle and its units are kJ/mol.
Factors Affecting Lattice Enthalpy.
- Ionic Radius – The lattice energy becomes less exothermic as the ionic radius of the ions increases because the charge on the ions is more spread out over the ion when the ions are larger. The ions are also further apart from each other in the lattice.
- Ionic Charge – The lattice energy gets more exothermic as the ionic charge of the ions increases and we know that the greater the ionic charge, the higher the charge density.This results in a stronger electrostatic attraction between the oppositely charged ions in the lattice and in turn, the lattice energy is more exothermic
Formula to Calculate the Lattice Enthalpy.
We can calculate the lattice energy of any ionic solid rather accurately by using a modified form of Coulomb’s law as shown below.
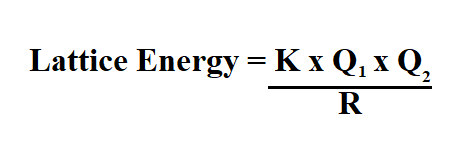
- K is the constant (2.31 x 10 ^ -19)
- Q1 is the numerical ion charge of ion 1.
- Q2 is the numerical ion charge of ion 2.
- R is the distance between ions.
Example:
Na Cl has an ion charge of +1 and -1, calculate the lattice energy if the distance between the two ions is 0.282 nm.
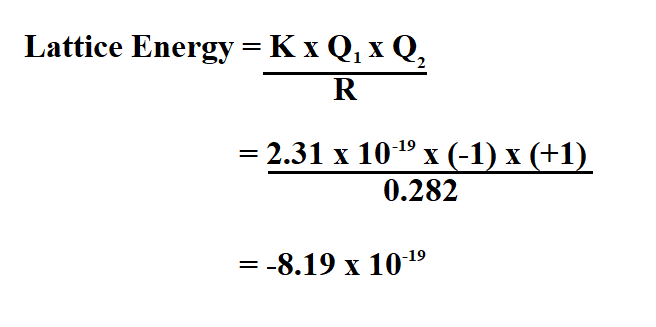
Therefore, the lattice energy is 8.19 x 10^-19kJ/mol. The negative sign of the energy is indicative of an exothermic reaction.

