Partial pressure is the pressure that a gas in a mixture of gases would exert if it occupied the same volume as the original mixture at the same temperature.
Partial pressure is extremely important in predicting the movement of gases.
Formula to calculate partial pressure.

Example:
A mixture of 2 mol of hydrogen gas and 3 mol of helium gas exerts a total pressure of 3 atm. What is the partial pressure of helium.
Since we know that mole fraction is;
= No. of moles of He ÷ Total no. of moles of the mixture
Therefore,
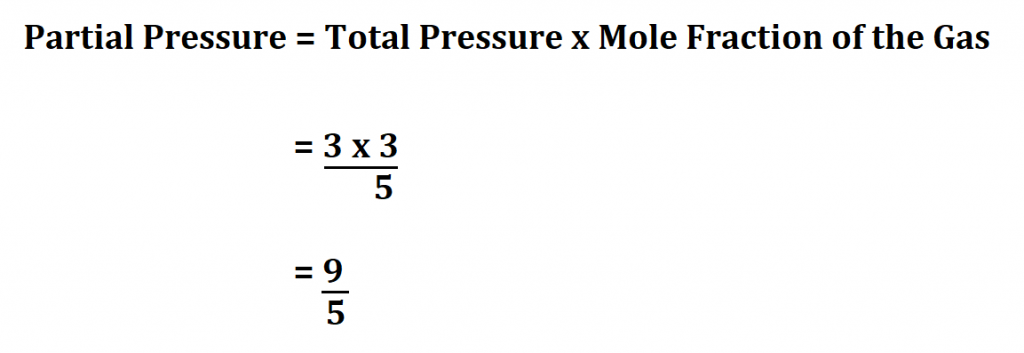
Therefore, the partial pressure of helium is 1.8 atm.