Kp stands for the equilibrium partial pressure.
It is defined as the partial pressures of the gasses inside a closed system.
It is used to express the relationship between product pressures and reactant pressures.
It is a unitless number, although it relates the pressures.
Formula to calculate Kp.
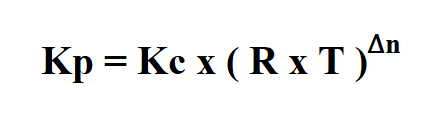
- Kc is the by molar concentration.
- R is the gas constant ( 0.08206 atm mol^-1K^-1, )
- T is gas temperature in Kelvin.
- Δn=mol of product gas−mol of reactant gas
Example:
Suppose the Kc of a reaction is 45,000 at 400K. What is the equilibrium constant at the same temperature if delta n is -2 mol gas .
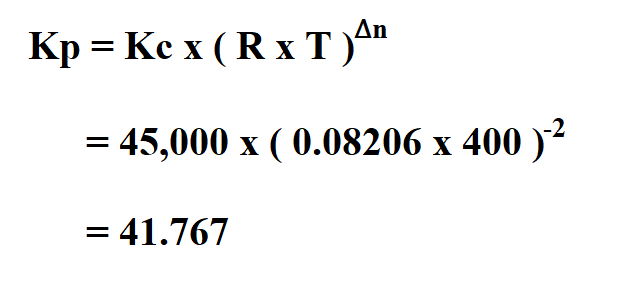
Therefore, the gas Kp is 41.767.