Rate constant is the proportionality constant in the equation that expresses the relationship between the rate of a chemical reaction and the concentrations of the reacting substances.
By finding out how fast products are made and what causes reactions to slow down we can develop methods to improve production.
Formula to calculate rate constant.
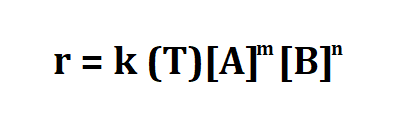
- r is the reaction rate.
- k(T) is the reaction rate constant that depends on temperature.
- [A] is the molar concentrations of substances A in moles per unit volume of solution.
- [B] is the molar concentrations of substances B in moles per unit volume of solution.
- m,n are the partial orders of reaction.
Example:
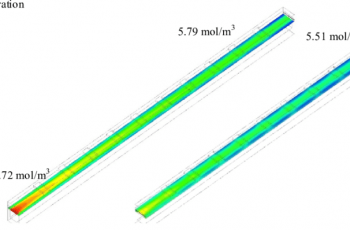
Suppose you are given the following figures that were extracted from the reaction of a certain substance.
- k(T) 1.0 x 10^2
- [A] 0.002 M
- [B] 0.001 M
- m 2
- n 0
So all we need to do here is plug in the value.

Therefore, the rate constant rate is 4 x 10^-4 M/s.