Mass percent is one way of representing the concentration of an element in a compound or a component in a mixture.
Mass percent can also be defined as the ratio of the mass of a specific element in a compound to the total mass of the compound.
Formula to calculate mass percent.
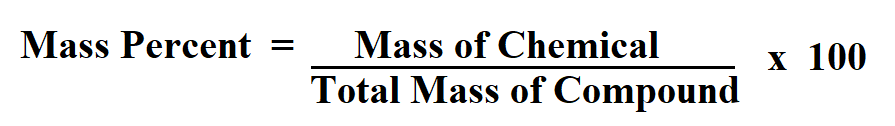
Mass of chemical is the mass of the chemical in question.
To calculate the total mass of the compound we sum the masses of all of the chemicals used to make the compound or solution.
Example:
Calculate the percent mass of 5g of sodium hydroxide dissolved in 100g of water?
The mass in question is 5g sodium hydroxide.
The total mass of the compound is;
100g + 5g = 105g
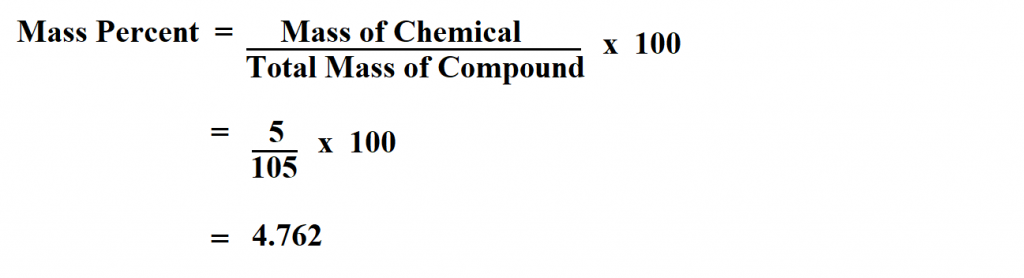
Therefore, the mass percent of NaOH is 4.762.