Ka is the acid dissociation constant while pH is the measure of the acidity or basicity of aqueous or other liquid solutions.
The dissociation constant for a strong acid can be as high as 10^7 while for a weak acid it can be as low as 10^-12 .
Formula to calculate Ka from pH.
To find Ka, you will need to use the ICE (Initial, Change, Equilibrium) table and the following formula.
To calculate Ka, we divide the concentration of the products by the concentration of the reactants.
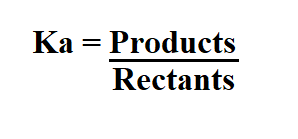
Example:
Calculate the Ka of 2M hypochlorus acid (HCIO) if its pH is 5.
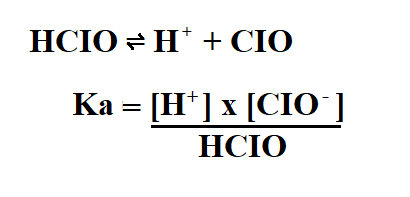
Then, we use the ICE table to find the concentration of the products.
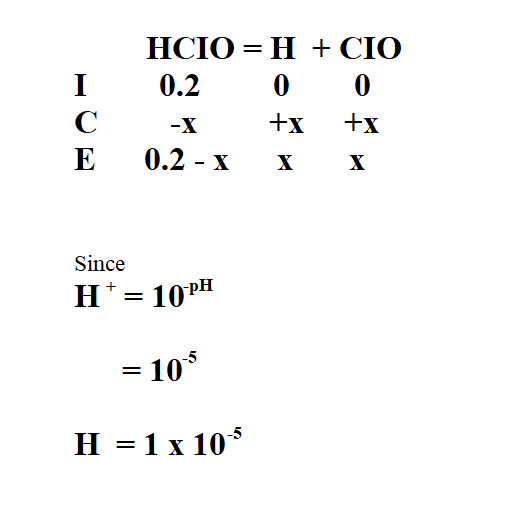
Therefore, x is 1 x 10^-5.
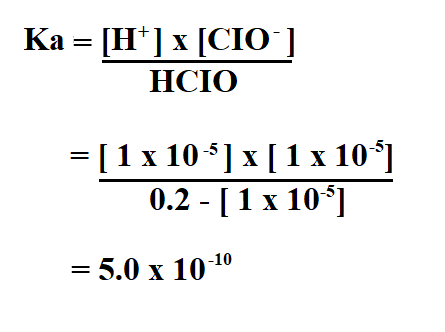
Therefore, the Ka of the hypochlorus acid is 5.0 x 10^-10.

