The mole or mol is an amount unit similar to familiar units like pair, dozen, gross, etc.
Mole, in chemistry, is a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
Formula to calculate moles from grams.
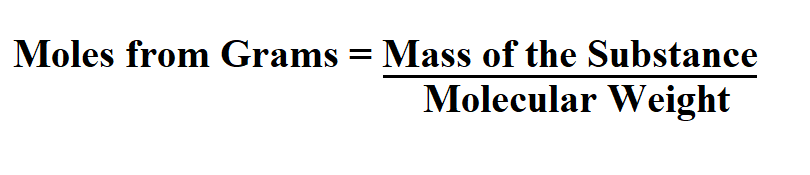
The mass of the substance must be in grams.
Example:
Suppose you have 16 grams of water. Calculate the moles from the grams.
Since water has two molecules of hydrogen and one molecule of oxygen, then the molecular weight of water is 18.01528g/mol. Therefore;
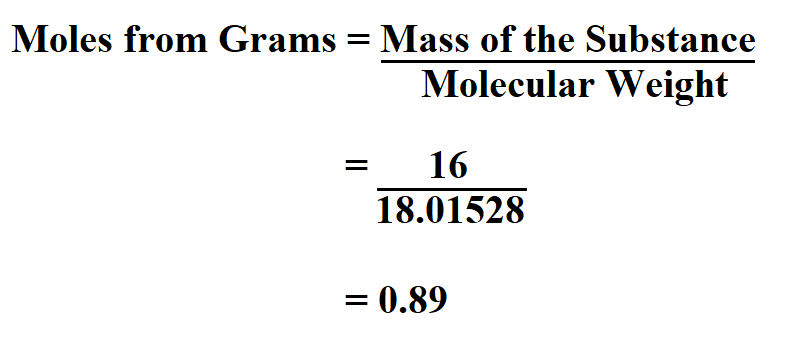
Therefore, for 16 grams of water there is 0.89 moles.