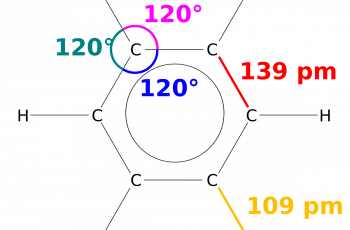
A dipole moment is a quantity that describes two opposite charges separated by a distance and hence its a vector quantity.
Dipole moments tell us, on average, where the electrons in a molecule are and they can also tell us the shape of molecules.
Formula to calculate dipole moment.

Example:
Calculate the dipole moment of a water molecule.
Since the bond moment of the O-H bond is -1.5 Debyes.
The H−O−H bond angle of water is pretty much 104.5 degrees. Then we divide 104.5 by 2 to get 52.25 degrees for each side, then imagine two right angles.
With that, and the fact that cosθ=cos(−θ).
Therefore, our left contribution will be;
= 1.5 x cos ( -52.25 )
= 0.918
And our right contribution will be the same as the left contribution.
= 0.918
Finally, we sum them up.
= 0.918 + 0.918
= 1.836 D