In this article we will discuss how to calculate concentration from absorbance but first lets begin by defining absorption.
Absorption means that a substance captures and transforms energy while concentration refers to the amount of a substance in a defined space.
If we increase the concentration of the solution then there are more molecules for the light to hit when it passes through.
Scientists use the Beer-Lambert Law also known as Beer’s Law in order to find concentration from absorbance.
The relationship between absorbance and concentration (c) is proportional. Usually, the more concentrated a substance, the more light will be absorbed. Thus the absorbance (A) of the material is related to the initial intensity of the light, I0, and the transmitted intensity of the light.
Formula to Calculate Concentration from Absorbance.
We can find concentration by plotting a graph of Absorbance against Concentration of known solutions. So if you subtract your y-intercept from the absorbance and divide by the slope, you are finding the concentration of your sample.
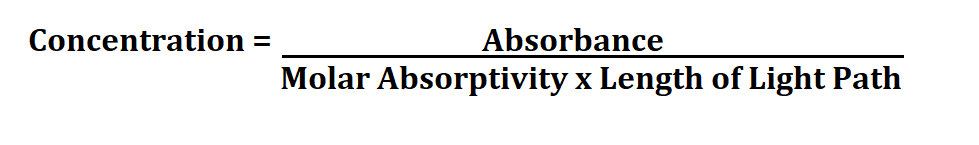
Example:
Suppose the molar absorptivity of Na Cl is 193L mol-1 cm-1 and the length of its light path is 5 cm, calculate the concentration if the absorbance is 200.

Therefore, the concentration is 0.21M.